Help patients
like Joshua
to participate
socially1
Xeomin is indicated for the symptomatic treatment
in children and adolescents aged 2 to 17 years
and weighing ≥ 12 kg of chronic sialorrhea due to
neurological/neurodevelopmental disorders.2
Efficacy
XEOMIN® showed significant reduction in unstimulated salivary flow rate from baseline vs. placebo for 16 weeks3
-
The co-primary efficacy endpoint for uSFR was reached demonstrating XEOMIN®’s
superiority vs placebo (age 6-17 yrs)
Mean changes in uSFR from baseline to study visit (main period)
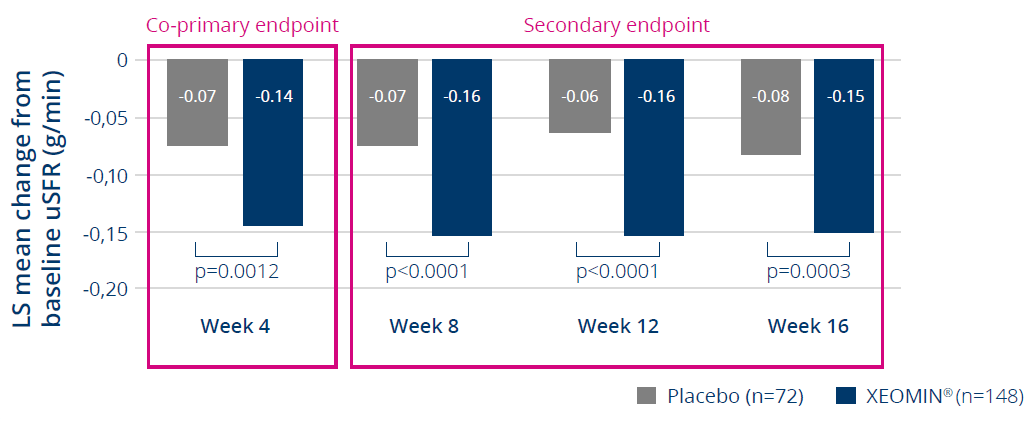
50% greater
reduction in
uSFR from
baseline with
XEOMIN®
treatment than
with placebo.
-
XEOMIN® significantly reduced uSFR vs. placebo at each 4-week assessment from
week 4 to week 16, with no substantial waning of effect seen for up to 16 weeks
LS, least squares; uSFR, unstimulated salivary flow rate.
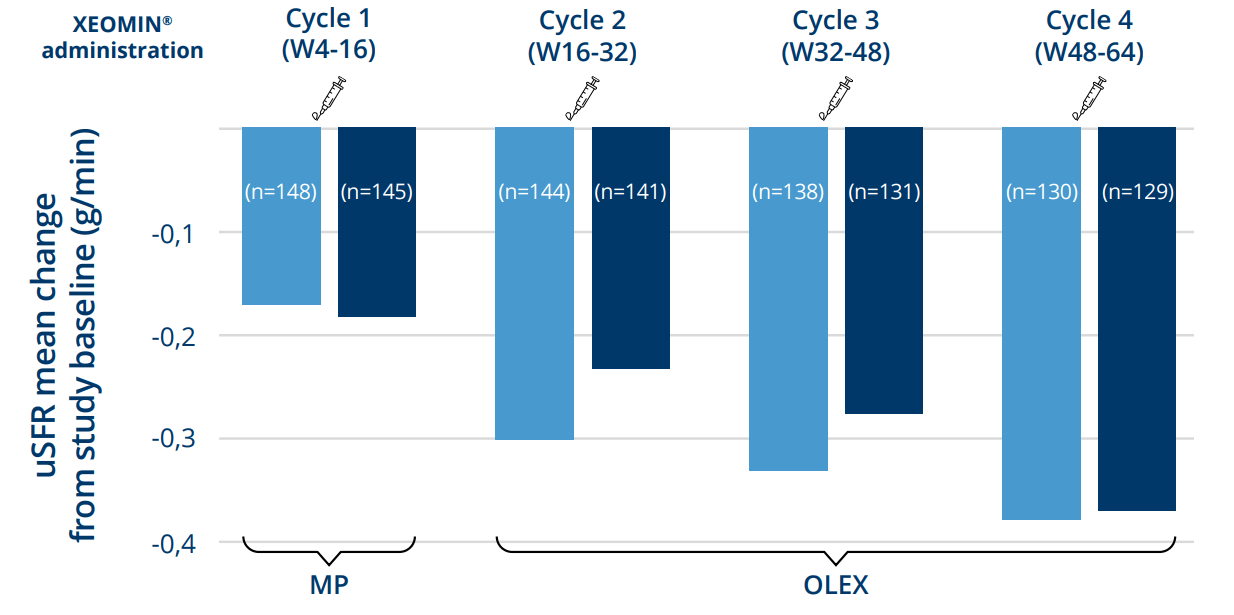
XEOMIN® resulted in cumulative improvement of sialorrhea with repeated injections up to 64 weeks3
Mean change from baseline in uSFR following repeated administrations
XEOMIN® (age 6-17 yrs)*
-
Rating at week 4 of follow-up cycle
-
Rating at week 16 of follow-up cycle
-
The outcomes for 2-5 year olds in the OLEX phase also showed a sustained effect over time3
*Data shown for patients age 6-17 years in the XEOMIN® treatment group.
MP, main period; OLEX, open-label extension; uSFR, unstimulated salivary flow rate; Wk, week.
Tolerability
XEOMIN® is well tolerated in children and adolescents aged 2-17 years with neurological / neurodevelopmental disorders for the treatment of chronic sialorrhea3
Treatment-emergent adverse events (main study phase)4,5
| Type of adverse event | Age 2-5 years | Age 6-17 years | |
|---|---|---|---|
| XEOMIN® (n=35) | XEOMIN® (n=148) | Placebo® (n=72) | |
| Any adverse event [n,(%)] | 5 (14.3) | 27 (18.2) | 11 (15.3) |
| Any treatment-related adverse event [n,(%)]* | 1 (2.9) | 1 (2.9) | 0 |
| Any serious adverse event [n,(%)] | 1 (2.9) | 0 | 1 (1.4) |
| Any treatment-related serious adverse event [n,(%)] | 0 | 0 | 0 |
*As assessed by clinical investigator.
-
Adverse events were mild to moderate3
-
No treatment-related serious adverse events were reported
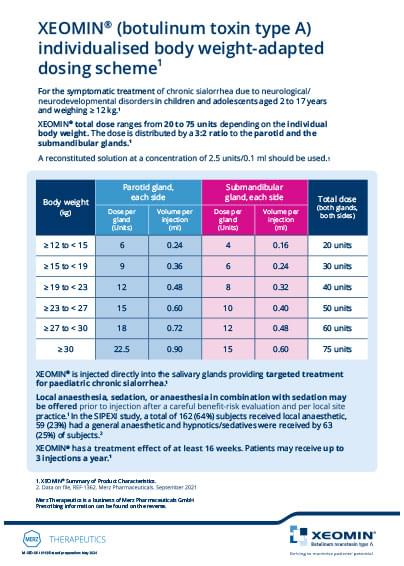
Dosing and administration
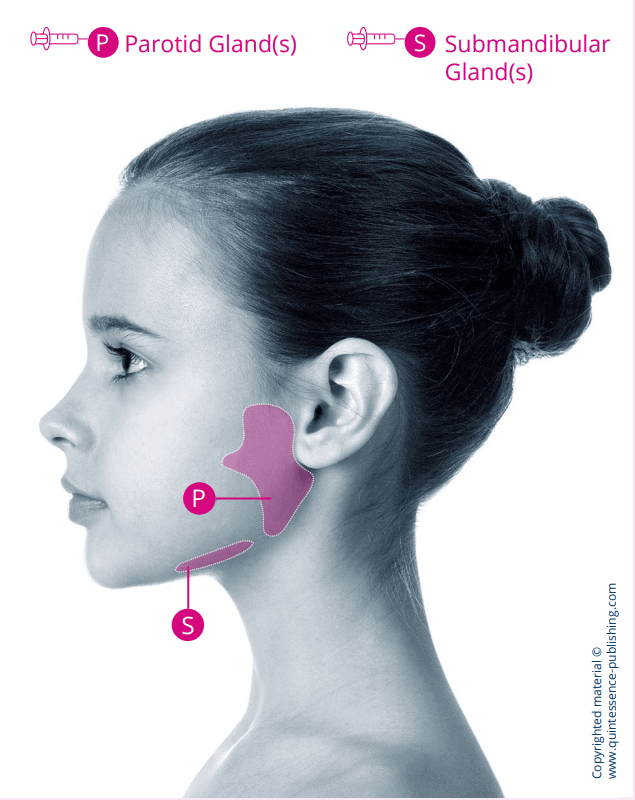
XEOMIN® is injected directly into the salivary glands, providing targeted treatment for paediatric chronic sialorrhea2
-
Total dose ranges from 20 to 75 units depending on the individual body weight. The dose is distributed by a 3:2 ratio to the parotid and the submandibular glands2
-
Ultrasound imaging should be used to guide needle placement into the salivary glands2
-
Local anaesthesia, sedation, or anaesthesia in combination with sedation may be offered prior to injection after a careful benefit-risk evaluation and per local site practice2
-
Treatment intervals should be determined based on the actual clinical need of the individual patient. Repeat treatment should be no more frequent than every 16 weeks2
XEOMIN® individualised weight adapted dosing scheme2
| Body weight (kg) |
Parotid gland, each side |
Submandibular gland, each side |
Total dose (both glands, both sides) |
||
|---|---|---|---|---|---|
| Dose per gland (units) |
Volume per injection (ml) |
Dose per gland (units) |
Volume per injection (ml) |
||
| ≥ 12 to < 15 | 6 | 0.24 | 4 | 0.16 | 20 units |
| ≥ 15 to < 19 | 9 | 0.36 | 6 | 0.24 | 30 units |
| ≥ 19 to < 23 | 12 | 0.48 | 8 | 0.32 | 40 units |
| ≥ 23 to < 27 | 15 | 0.60 | 10 | 0.40 | 50 units |
| ≥ 27 to < 30 | 18 | 0.72 | 12 | 0.48 | 60 units |
| ≥ 30 | 22.5 | 0.90 | 15 | 0.60 | 75 units |
Local anaesthesia, sedation, or anaesthesia in combination with sedation may be offered prior to injection after a careful benefit-risk evaluation and per local site practice.2 In total 162 (64%) subjects received local anaesthetic, 59 (23%) had a general anaesthetic and hypnotics/sedatives were received by 63 (25%) of subjects.6
| ATC Class Level 3 |
Placebo group (6-17 years) (N=72) n (%) |
Xeomin group (6-17 years) (N=148) n (%) |
Xeomin group (2-5 years) (N=35) n (%) |
|---|---|---|---|
| Local Anaesthetic | 42 (58.3) | 95 (64.2) | 25 (71.4) |
| General Anaesthetic | 17 (23.6) | 38 (25.7) | 4 (11.4) |
| Hypnotics and sedatives | 16 (22.2) | 41 (27.7) | 6 (17.1) |
Resources
Contact Us
please contact Merz Therapeutics UK & Ireland on 0333 200 4143 or [email protected]
To register for training events please complete the form below and submit here
By emailing and registering my data with Merz, I acknowledge that Merz Therapeutics may process my personal data in accordance with their privacy policy. I also acknowledge that Merz Therapeutics may use my personal data to contact me via email for marketing purposes.
What’s NEXT?
What is your goal with your patients?
Let’s take BoNT therapy to the next level, together.
- Networking therapists - Promoting cooperation between therapists and BoNT injectors
- Education - High quality injection training with internationally recognised trainers
- XEOMIN® - Botulinum neurotoxin free from complexing proteins
- Team - Committed to working together to improve patient outcomes